When it comes to understanding the boiling point, it's essential to grasp the concept in the context of temperature measurements. The boiling point refers to the temperature at which a liquid turns into vapor, and it varies depending on the atmospheric pressure. In the United States, the Fahrenheit scale is commonly used, making it crucial to understand the boiling point in Fahrenheit (F) for various substances. Whether you're a student, a scientist, or just curious, knowing the boiling point in F can provide valuable insights into the properties of different materials.
Boiling points are not just a matter of scientific curiosity; they have practical applications in everyday life and industrial processes. For example, the boiling point in F is critical in cooking, where water's boiling point is around 212°F under standard atmospheric pressure. Beyond cooking, industries rely on boiling points to distill liquids, such as in the production of alcohol and petroleum refining. Understanding the boiling point in F can also help in weather prediction, as changes in atmospheric pressure affect boiling temperatures.
In this comprehensive article, we will delve into the concept of boiling points with a focus on Fahrenheit measurements. We will explore the scientific basis of boiling points, examine the factors that influence them, and discuss their significance in various fields. Additionally, we'll answer common questions about boiling points and provide a detailed guide to understanding this fundamental scientific concept. So, whether you're a student, a professional, or just someone with a keen interest in science, this article is tailored to enhance your understanding of boiling points in F.
Read also:Hassan Jameel And Rihanna A Story Of Love Business And Global Influence
Table of Contents
- What is the Scientific Basis of Boiling Points?
- Factors Influencing Boiling Point in F
- Why is the Boiling Point in F Important?
- What are Standard Conditions for Boiling Points?
- The Boiling Point of Water in F
- Comparing Boiling Points of Different Substances
- What is Boiling Point Elevation?
- Applications of Boiling Points in F
- Techniques for Measuring Boiling Points
- How Does Pressure Impact Boiling Points?
- Safety Considerations with Boiling Points
- Boiling vs. Vaporization: What's the Difference?
- Understanding Boiling Point Diagrams
- Frequently Asked Questions
- Conclusion
What is the Scientific Basis of Boiling Points?
The boiling point is a fundamental concept in thermodynamics and physical chemistry, representing the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. At this point, the liquid changes into vapor. The scientific principle underlying boiling points is rooted in the kinetic theory of matter, which explains how particles move and interact.
As a liquid is heated, its molecules gain kinetic energy and begin to move more rapidly. When the temperature reaches the boiling point, the molecules have enough energy to overcome intermolecular forces, transitioning from the liquid phase to the gaseous phase. This process occurs when the vapor pressure of the liquid equals the atmospheric pressure.
It's important to note that boiling points vary based on external pressure. At higher altitudes, where atmospheric pressure is lower, liquids boil at lower temperatures. Conversely, in a pressurized environment, the boiling point increases. This variability makes understanding boiling points in F crucial for different geographical and industrial contexts.
Factors Influencing Boiling Point in F
Several factors can influence the boiling point of a substance, particularly when measured in Fahrenheit. These factors include:
- Intermolecular Forces: Substances with strong intermolecular forces, such as hydrogen bonds, tend to have higher boiling points. This is due to the extra energy required to break these forces during the phase transition.
- Molecular Weight: Generally, compounds with higher molecular weights have higher boiling points because they have more mass and often more surface area for intermolecular interactions.
- External Pressure: As mentioned, the boiling point is directly related to atmospheric pressure. At lower pressures, the boiling point decreases, and at higher pressures, it increases.
- Purity of the Substance: Impurities can either raise or lower the boiling point, depending on the nature of the impurity and its interaction with the main substance.
- Type of Liquid: Different liquids have different boiling points due to their unique chemical structures and intermolecular forces.
Understanding these factors is essential for accurately predicting and manipulating boiling points in various scientific and industrial applications.
Why is the Boiling Point in F Important?
The boiling point in F is a critical parameter in both scientific research and practical applications. Here's why it's important:
Read also:What Airport Is Dca Ndash A Comprehensive Guide To Ronald Reagan Washington National Airport
- Cooking and Food Processing: Knowing the boiling point of water and other liquids is essential for preparing food safely and efficiently. It affects cooking times and methods, such as boiling, steaming, and blanching.
- Chemical Engineering: In chemical industries, the boiling point is crucial for designing distillation processes, where separating components based on their boiling points is necessary.
- Environmental Science: Understanding boiling points helps in studying weather patterns and environmental changes, as temperature and pressure affect the boiling of water bodies.
- Safety and Hazard Management: Boiling points are used to assess the volatility of substances, which is important for storage and handling safety.
Boiling points also play a significant role in the pharmaceutical industry, where the stability of liquid medications is often dependent on their boiling points.
What are Standard Conditions for Boiling Points?
Standard conditions for boiling points refer to a set of baseline parameters under which the boiling point of a substance is measured and reported. These conditions are typically defined as:
- Standard Atmospheric Pressure: This is usually 1 atmosphere (atm) or 101.325 kilopascals (kPa).
- Standard Temperature: The temperature at which the measurement is taken, often considered to be 25°C (77°F) for convenience.
Under these standard conditions, water boils at approximately 212°F. Deviations from these conditions require adjustments in boiling point measurements to account for changes in atmospheric pressure or temperature.
The Boiling Point of Water in F
Water is one of the most commonly referenced substances when discussing boiling points. Under standard conditions, the boiling point of water is 212°F. However, this can change based on atmospheric pressure.
At higher altitudes, where atmospheric pressure is lower, water boils at a temperature lower than 212°F. For example, in Denver, Colorado, which is approximately 5,280 feet above sea level, water boils at about 202°F. This has implications for cooking and other processes that involve boiling water.
Conversely, in a pressurized environment, such as a pressure cooker, water can boil at temperatures higher than 212°F. This phenomenon is utilized in cooking to prepare food faster by increasing the boiling temperature.
Comparing Boiling Points of Different Substances
Various substances have distinct boiling points, which can be compared to understand their physical properties. For instance:
- Ethanol: Has a boiling point of around 173°F, lower than water, making it more volatile.
- Acetone: Boils at approximately 133°F, indicating even higher volatility compared to ethanol.
- Mercury: With a boiling point of 674°F, mercury remains liquid at much higher temperatures compared to other common liquids.
These differences are primarily due to the varying intermolecular forces and molecular structures of these substances. Understanding these differences is crucial for applications such as distillation, where separation relies on boiling point disparities.
What is Boiling Point Elevation?
Boiling point elevation refers to the phenomenon where the boiling point of a liquid increases when a solute is dissolved in it. This is a colligative property, meaning it depends on the number of solute particles rather than their identity.
When a non-volatile solute is added to a solvent, it disrupts the solvent's normal ability to vaporize, requiring more energy (higher temperature) to reach the boiling point. This is observed in everyday scenarios such as adding salt to water, which raises its boiling point.
Boiling point elevation is significant in industries like food processing and chemical manufacturing, where controlling boiling points is crucial for product quality and efficiency.
Applications of Boiling Points in F
The concept of boiling points extends beyond the laboratory, playing a vital role in various real-world applications:
- Cooking and Culinary Arts: Understanding boiling points helps chefs and cooks adjust recipes and techniques for different altitudes and cooking methods.
- Pharmaceuticals: Boiling points are used to ensure the stability and efficacy of liquid medications, as some active ingredients may degrade at certain temperatures.
- Industrial Processes: In industries like petroleum refining, knowing the boiling points of different hydrocarbons is essential for efficient distillation and separation.
- Environmental Monitoring: Boiling points help in predicting evaporation rates and their impact on weather patterns and climate change.
These applications highlight the importance of understanding boiling points in F for both practical and scientific endeavors.
Techniques for Measuring Boiling Points
Accurate measurement of boiling points is crucial for scientific research and industrial applications. Here are some common techniques:
- Distillation: One of the oldest methods, where a liquid is heated, vaporized, and then condensed to measure the temperature at which it boils.
- Boiling Point Apparatus: Specialized equipment designed to precisely measure the boiling point of a liquid under controlled conditions.
- Dynamic Method: Involves heating the liquid in a closed system and recording the temperature at which it transitions to vapor.
Each technique has its advantages and limitations, and the choice of method depends on the specific requirements of the measurement and the properties of the substance being studied.
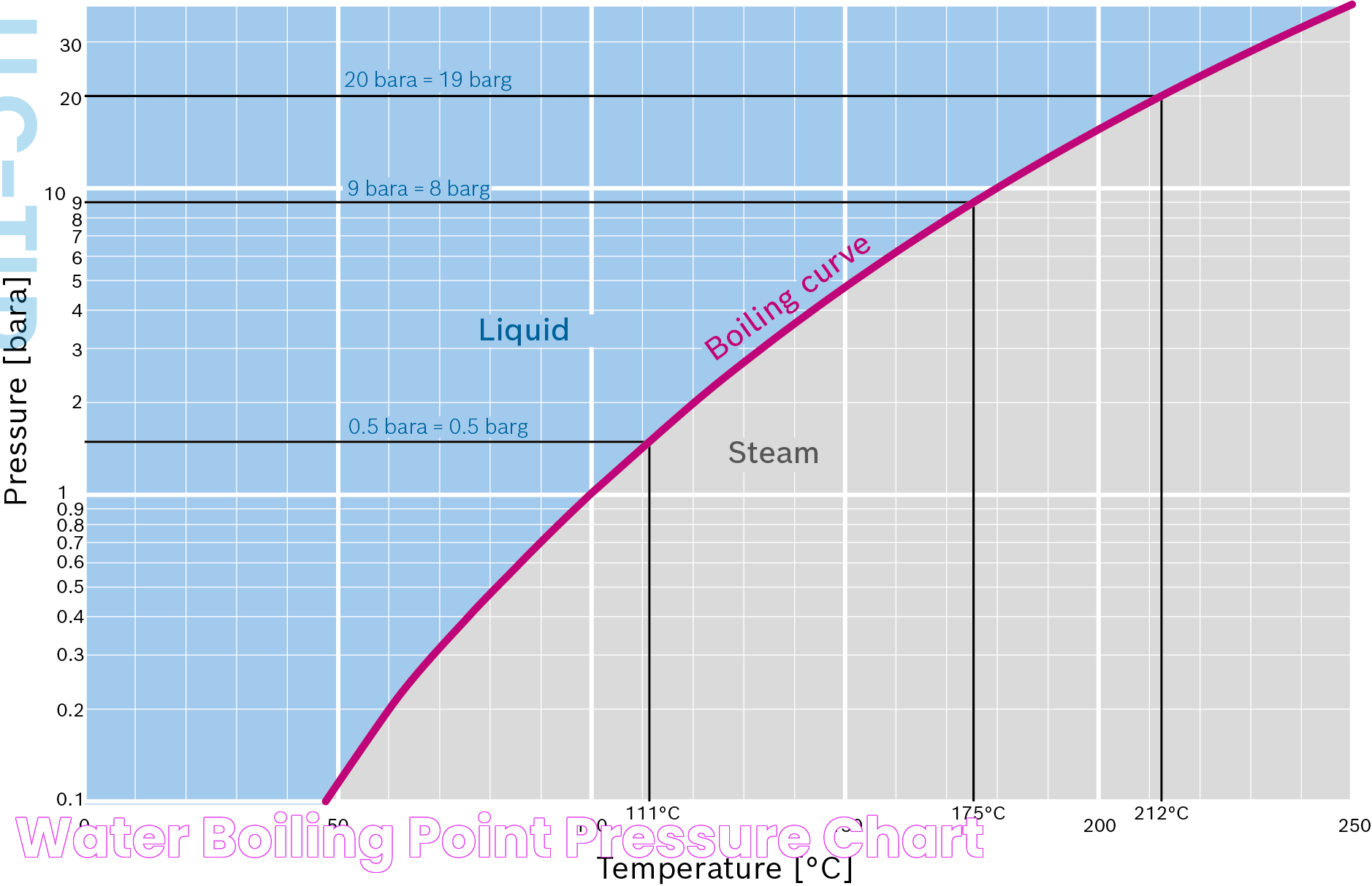
How Does Pressure Impact Boiling Points?
Pressure has a direct and significant impact on boiling points. As pressure increases, the boiling point of a liquid also increases. Conversely, as pressure decreases, the boiling point drops. This relationship is crucial for understanding and predicting boiling behavior under different atmospheric or experimental conditions.
For example, in a pressure cooker, the increased pressure raises the boiling point of water above 212°F, allowing food to cook faster. On the other hand, at high altitudes, the decreased pressure lowers the boiling point, requiring adjustments in cooking times and techniques.
This pressure-boiling point relationship is also exploited in industrial applications, where controlling pressure allows for precise temperature regulation during chemical reactions and processes.
Safety Considerations with Boiling Points
Understanding boiling points is essential for ensuring safety in both laboratory and industrial settings. Here are some key considerations:
- Volatility and Flammability: Substances with low boiling points are often more volatile and can pose flammability risks. Proper storage and handling are essential to prevent accidents.
- Pressure Vessels: When working with pressure vessels, it's crucial to monitor and control pressure to avoid exceeding the boiling point, which can lead to explosions.
- Thermal Stability: Knowing the boiling point helps in assessing the thermal stability of materials, preventing degradation or hazardous reactions.
These safety considerations underscore the importance of accurate boiling point measurements and understanding for safe and effective material handling.
Boiling vs. Vaporization: What's the Difference?
Boiling and vaporization are often used interchangeably, but they refer to different processes:
- Boiling: Occurs when a liquid turns into vapor at its boiling point, with vaporization happening throughout the entire liquid.
- Vaporization: A broader term that includes boiling but also covers evaporation, where a liquid turns into vapor at temperatures below its boiling point, typically occurring on the liquid's surface.
Understanding the distinction between these terms is important for accurately describing and predicting phase changes in various contexts.
Understanding Boiling Point Diagrams
Boiling point diagrams are graphical representations that show the relationship between temperature, pressure, and composition for binary mixtures. These diagrams are valuable tools for understanding boiling behavior and designing distillation processes.
In a typical boiling point diagram, the x-axis represents the composition of the mixture, while the y-axis represents temperature. The diagram shows the boiling points of both components and the mixture at different compositions and pressures.
Boiling point diagrams are essential for industries that rely on separation processes, such as petrochemical and pharmaceutical industries, where precise control over boiling points is crucial for product quality and efficiency.
Frequently Asked Questions
What is the significance of knowing the boiling point in F?
Knowing the boiling point in F is crucial for various applications, from cooking to industrial processes, as it helps predict phase changes and control reactions.
How does altitude affect the boiling point in F?
At higher altitudes, atmospheric pressure is lower, causing the boiling point in F to decrease, which can affect cooking times and methods.
Can boiling point in F be altered?
Yes, boiling points can be altered by changing atmospheric pressure or adding solutes, which can raise or lower the boiling point depending on the situation.
Why is boiling point elevation important?
Boiling point elevation is important for understanding how solutes affect liquid behavior, which is critical for industries like food processing and chemical manufacturing.
Are boiling points the same for all liquids?
No, boiling points vary widely among different liquids due to differences in intermolecular forces, molecular weight, and other physical properties.
How is boiling point measured?
Boiling point is typically measured using techniques like distillation, boiling point apparatus, or dynamic methods, depending on the substance and application.
Conclusion
Understanding the boiling point in F is a fundamental aspect of both scientific inquiry and practical application. From cooking and industrial processes to environmental science and safety considerations, boiling points play a crucial role in our daily lives. By exploring the factors that influence boiling points, the methods for measuring them, and their wide-ranging applications, we gain valuable insights into the physical properties of substances and how they interact with their environment. Whether you're a student, a professional, or simply curious, a solid grasp of boiling points in F can enhance your understanding of the world around you.